“Us primary [kids] are much brighter than grown ups think! We hear about atoms all the time on the Big Bang Theory and The Simpsons, so why have they decided to keep atoms a secret in primary school?” – Atomic Kids (2013)
Well, the secret is no more in BC! I must admit, that last year when I started to explore the new curriculum I was astounded to find “all matter is made of particles” and “atoms are building blocks of matter” in grade 3 science (“Building Student Success,” 2016). I was also intimidated. Through a lot of exploration with my class I was blown away to see how captivated and interested they were in the subject. I created this visual to showcase how I would integrate T-GEM into my approach with this topic:
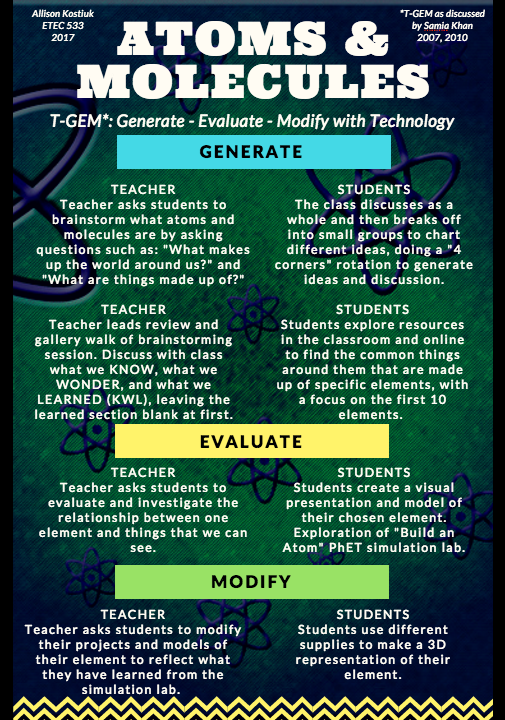
Atoms & Molecules with T-GEM by Allison Kostiuk
I found a PhET simulation lab for “Build an Atom” that I would utilize. Funny I did not realize I had found a “simulation lab” last year until I revisited it again through my class blog. I found that this resource helps my students “visualize aspects of science that are…too large [and] too small…to view” (Khan, 2010, p. 216).
Atomic Kids. (2013). Retrieved March 03, 2017, from http://atomickids.org/
Building Student Success – BC’s New Curriculum. (2016). Retrieved March 03, 2017, from https://curriculum.gov.bc.ca/curriculum/science/3
Khan, S. (2010). New pedagogies for teaching with computer simulations. Journal of Science Education and Technology, 20(3), 215-232.
P.S. If you are looking for a cute song related to this topic, The Atoms Family is a great one!
Hi Allison,
Great infographic for T-Gem. Also, love the name of the unit (well except I can’t get the tv jingle out of my head now). I really like the modify step of your process. Having students correct previous errors based on new found knowledge, in my opinion, often negates the old misconceptions and builds new pathways of understanding. Correcting misconceptions is so important. It always drove me crazy when my kids would have a test and they would write down the right answers during the “take up” session but they had no idea how or why those answers were correct. Taking the time to make these discoveries is so important and yet it falls prey to the usual reasoning by staff “there is no time to do that, I have so much to cover”. Is it any wonder students who do poorly on a specific unit also then do poorly on that section of the exam?
Catherine
Allison,
I think your T-GEM is a great way for students to start tackling the rather abstract concept of atoms and subatomic particles. The Generate portion that has students share their ideas on what matter is made off and the various elements they know in a gallery walk and discussion is helpful because it imparts the concept that science is very much a collaborative experience, where everyone contributes ideas and refines them as a group.
I particularly liked the simulation because it allows students to view the relationship between elements and the number of protons or neutrons they have, with the option of viewing various characteristics such as ion charge or stability. Curiously, I put together a Helium ion with 5 electrons and the simulation still stated that it was stable. Particle physics is not my specialty but my gut tells me that is incorrect?
Regardless, for the grade level, the simulation will be very helpful in having students connect the world around them with the concept of matter and a way to visualize the organization of the atom. Their understanding now will be very useful as they revisit and revise these concepts in their later years (such as transition out of Bohr models and into electron orbitals).
A particularly strong element of your design, in my opinion, is that in the evaluate phase, you have students create a digital model, and then in the modify phase, you have students create a tangible model. This seems to be an effective way to ensure that students are able to transfer their findings from the simulation, as well as to engage both the technologically-minded students and the hands-on kinesthetic learners. It would be interesting to see what the students would choose to make their tangible models out of!
Hi Allison,
The infographic is a very captivating way to present your lesson on atoms and molecules in grade 3. Thank you for producing it and applying your lesson to your new curriculum requirements. With the new curriculum in BC, elementary (and soon secondary) teachers in BC are having to introduce new topics and write new lessons for their classes in science.
Can you tell us more about your 4 corners activity? Also, when students are invited to imagine an element and what can they see, what have their responses been (or if you haven’t done the lesson, what might they be expected to say?
The PhEt simulation shows that atoms are made up of parts, but in the case of Li, one can add many electrons while keeping the protons the same (3). This might be an interesting opportunity to discuss their different models with students even though the element bears the same name. Samia
Hi Samia,
A 4 corners activity is when you split the class up evenly to the four corners of the classroom. In this case, the brainstorming paper would remain in the corner and each group would have a set time to add their thoughts to it before rotating to the next corner.
When I introduced the topic of atoms and molecules last year to my grade 3’s and asked them to think about any elements they know, their answers ranged from “air”, “water”, and then recognizing “gold” and “silver”. When pressed about water, many students knew H2O, but not necessarily what that meant.
I agree with you that the simulation brings about opportunities to discuss ions and such. For most students this is way over their head at this level, but I found it worthwhile introducing it to them for the handful who were able to comprehend it. When I taught this topic last year (I have grade 3 this year but am teaching computers to other classes instead of science in my classroom which another teacher comes in to teach), I had 17 active boys and 7 girls. It will be interesting to compare their enthusiasm and interest to other classes I teach this topic in the future.
Allison