While the ideas behind Technology-enhanced Generate-Evaluate-Modify (T-GEM) were not new to me on their own, this particular model of thinking, leading to inquiry and ultimately a deeper understanding of concepts taught, was. I appreciated the step-by-step process by which GEM takes both educators and students through the learning process of generating, evaluating and modifying ideas; a model which I believe highlights a weakness in my own teaching of science and math. For me, one of the most important points to consider was the cyclical pattern in which GEM encourages students to create new hypotheses as they generate, examine, and evaluate new and existing data, and then re-examine and modify relationships between data and ideas/hypotheses generated (Khan 2007 & 2010). I believe I often struggle with time constraints (either real or perceived/self-imposed) which means that I miss important steps in this learning cycle. Going forward, I believe that having a model like T-GEM will help me focus my planning on creating a more inquiry-based environment in my own classroom. As highlighted by a survey item generated by Khan, students overwhelmingly felt that “having us generate, evaluate, and modify relationships in class is valuable for my understanding of the concepts” taught (in this case referring to chemistry) with 91% of students agreeing with the statement, 0% disagreeing, and 9% neutral (Khan, 2007, p.900 – included percentages; Khan, 2010, p. 227 – listed as a survey question only). While this particular survey question related to a university-based chemistry course, I believe it is true for any science-based concept that students are required to learn.
The simulations in Chemland were not applicable to my current students as I work in a grade 4/5 split class, but I enjoyed exploring the program and I recognize the incredible possibilities the simulations would provide for an appropriate age/grade group. Having said that, I could see the “Specific Heat Capacity” simulation (http://employees.oneonta.edu/viningwj/sims/specific_heat_s.html) and even “Heat Transfer Between Substances” (http://employees.oneonta.edu/viningwj/sims/heat_transfer_s.html) having the potential to be used by at least some students in a higher-level intermediate grade within an elementary school (i.e., grade 6/7).
T-GEM Model with assignment (aimed at grade 4 Science):
For this activity, I chose to focus on evaporation due to the fact that students often have a variety of misconceptions related to “phase changes of water” (p. 4), as is identified by Laura Henriques (2000) in “Children’s misconceptions about weather: A review of the literature.” Henriques identifies that rather than understanding “water left in an open container evaporates changing from liquid to gas,” children may believe the water “is absorbed by the container” or simply “disappears (Bar, 1989; Osborne & Cosgrove, 1983);” that it “changes into air or disappears and turns into air ((Bar, 1989; Brody, 1993; Lee, et al., 1993; Osborne & Cosgrove, 1983);” or that “the water dries up – it is not steam, it just dries up and goes into the air (Bar, 1989)” (p. 5). Henriques points out that “all the misconceptions here (except water being absorbed by the container) are basically true since water vapor is a legitimate component of air,” however, students generally “were not viewing the evaporated water as a component of air because air to them is nothingness” (p. 5).
G – Generate:
To access prior knowledge, check for misconceptions, and generate ideas about the topic, evaporation, I would begin with a few different activities to activate thinking and knowledge from different angles.
1) First, students would complete a “What-So-What?” activity. Students would be given a handout with a t-chart on it. The left column of the chart says “What?” and the right column says “So What?” Students would be shown two pictures and would be expected to respond on “What” they see and why each thing they see might be of importance (“So What?”). The pictures will be projected through a projector/proxima to a screen for them. The first picture would be of a bowl/container filled with water, perhaps sitting near a window or outside on a sunny day. The students would be given a set amount of time (I usually allow only one minute) to list as many things as possible that they see in the picture. They are then given additional time (I usually give about two minutes) to respond to why each thing they saw might be important in the “So What?” column. Next, students would be presented with a picture of the same container, except this time it would be empty (time and weather could change, but container and its location would remain the same). They would repeat the “What-So-What?” process. I would then do a “Whip Around” to have each student share one idea they came up with from the pictures – students are allowed to expand on an idea already shared by a peer, but must make it “their own.”
2) Originally, I had thought that I would have students generate ideas they had about the term “evaporation” by doing an idea web on paper. However, having read Khan’s (2012), “A Hidden GEM: A pedagogical approach to using technology to teach global warming” I decided I would borrow an idea from Khan’s generate strategies and use the Cmap program discussed in the article. Cmap software was developed through research by the Florida Institute for Human & Machine Cognition (IHMC) and “empowers users to construct, navigate, share and criticize knowledge models represented as concept maps” (IHMC, 2014). It is marketed as a software that can be used by all age groups, as individuals, as well as within schools and institutions (http://cmap.ihmc.us/). I had never used Cmap before, but found it very easy to download and to use at basic level. I feel that my grades 4/5 students would be comfortable using this application.
Using Cmap, I would ask students to create an initial map of ideas they can generate about their beliefs related to evaporation. This would likely include ideas they generated through the “What-So-What” activity, from the “Whip Around” activity, as well as from their own prior knowledge. I would allow students to work in partners to encourage discussion about evaporation and ideas generated. Students would create a basic Cmap at this point.
For example:
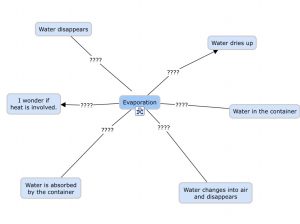
At this point, students would be asked (with their partner) to create a hypothesis based on the question: “What happens to water when it is left in an open container?”
(N.B. “What-So-What?” is a strategy I use quite often with my students, especially in Science and Social Studies lessons. I do not have an exact reference for this strategy, but it was shared by Faye Brownlie during a presentation she gave when visiting the district where I work a couple of years ago. The following is a link to her website: http://fayebrownlie.ca/)
E – Evaluate:
When I began searching for interactive simulations online, I found an interactive evaporation simulation that seemed to fit well with both the grade level I am aiming at (primarily grade 4) and the curriculum content that students will be expected to know “Solids, liquids and gases as matter” (B.C. Ministry of Education, 2016). Working in pairs to allow for discussion, students would use the online evaporation simulation to evaluate the ideas they generated in their Cmap. Students will be able to experiment with the following variables: how humid their environment will be (full sun, sun with clouds, clouds with rain), air temperature (between 10 and 40 degrees Celsius), and container shape (flatter and wider versus taller and thinner).
As students progress through the simulation, they will record/graph their findings taking into account the changes in variables.
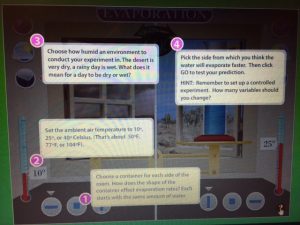

Interactive evaporation simulation link:
http://archive.fossweb.com/modules3-6/Water/activities/evaporation.html
M – Modify:
Once they have completed the interactive evaporation simulation and recorded their findings, students will return to their original Cmap webs to modify their original webs. New ideas/findings/knowledge can be added to the existing Cmap, and new concepts can be added. In addition to this, new connections can be made, connecting existing ideas together, and causal relationships can be identified, all within the Cmap.
If I am looking for a more in-depth assessment or for further evidence of learning, I could ask students to explain orally (conference-style) what they observed happening during the simulation and to explain the concepts and connections shown on their Cmap. I could also ask students to write a paragraph to explain what they have learned and/or to draw a picture showing their understanding. Once modifications to their Cmap are complete, students would be asked to modify their original hypothesis, based on the data they have collected (based again on the question: “What happens to water when it is left in an open container?”).
References:
British Columbia Ministry of Education (2016). Area of learning: Science, grade 4. Retrieved from https://curriculum.gov.bc.ca/sites/curriculum.gov.bc.ca/files/pdf/s_learning_standards.pdf
Henriques, L. (2000, April). Children’s misconceptions about weather: A review of the literature. Paper presented at the annual meeting of the National Association of Research in Science Teaching, New Orleans, LA. Retrieved 7 January, 2017, from: http://web.csulb.edu/~lhenriqu/NARST2000.htm
IHMC. (2014). Cmap. Retrieved from http://cmap.ihmc.us/
Interactive evaporation simulation (n.d.). Retrieved 27 February, 2017, from http://archive.fossweb.com/modules3-6/Water/activities/evaporation.html
Khan, S. (2012). A Hidden GEM: A pedagogical approach to using technology to teach global warming. The Science Teacher, 79(8), 59-62.
Khan, S. (2010). New pedagogies for teaching with computer simulations. Journal of Science Education and Technology, 20(3), 215-232.
Khan, S. (2007). Model-based inquiries in chemistry. Science Education, 91(6), 877-890
Dear Mary,
Your analysis of the GEM research was augmented with the use of links from the Chemland suite and raw data from the study. The use of some sample raw data assists readers in evaluating the general interpretations of the authors. Henriques review of children’s misconceptions that you offered revealed several insights into how we think about evaporation, air, and water. Research can affirm our observations and reveal children’s misconceptions that are not detected by our local assessments but nonetheless persist in influencing students’ classroom responses and their approaches to framing a problem in our classes. A few from my own research of grade fours and sevens revealed that students reported that hot particles leave (first) cold particles stay (or leave later) on evaporation or a pop can is sweating to explain condensation on a pop can.
In a follow up study on teachers (entitled What’s missing in model-based teaching), the most common missed steps are E and M. That is, teachers in the study were observed encouraging students to generate a model but not evaluate it again or return to it and modify.
Towards this, the T chart you suggested with this lesson would serve nicely to provide an environmental scan of what students’ know at the beginning of the lesson. Notable here (and in the chemistry case study) is that you do not correct students during the “Whip Around” in this first phase; all ideas are accepted. The concept map is a great way to see changes in students’ expressed models at the beginning and end of instruction. I’m glad to see you try John Novak’s concept mapping software (developed the idea with others of concept mapping), Cmap. New ideas/findings/knowledge can be added to the existing Cmap. Dr. Novak contacted me when my own work utilized the software, and I was happy to learn he was well into his eighties and keen on seeing how people use concept maps. Cmaps might be different from Kidspiration and other concept mapping software in that there is the capacity to collaborate world wide on concept maps. The question as well: “What happens to water when it is left in an open container?” invites students to apply their modified relationships or rules to a new problem.
Many assumptions and relationships built into students’ conceptual models of a phenomenon in science and your thoughtful plan encourages students to express these relationships, grapple with new information and each other’s ideas, and attempt to apply what they have learned; this is a well integrated TELE. Can you share a bit about whether students in your curriculum are expected to know about the particulate nature of matter and whether they would also be expected to represent evaporation in these terms or are the goals towards more of a macromolecular view of the process?
Thank you, Samia
Hi Dr. Khan,
Thank you for your response and feedback. I am not teaching science to my students this year, so I will not be able to try an interactive simulation like this with my class until next year and any planning based on the new curriculum that I am currently doing has not yet been used in the classroom, which is unfortunate. The new curriculum itself gives little guidance or clear learning expectations as far as the phase changes of matter, which means the interpretation will change between schools and teachers. The following information is what is provided for teachers to base their teaching on:
The “Big Idea” in the new Science 4 curriculum is that “Matter has mass, takes up space, and can change phase” with the elaborations being given in the form of sample questions to promote inquiry:
How can you explore the phases of matter?
How does matter change phases?
How does heating and cooling affect phase changes?
In terms of content, the new Science 4 curriculum says only “phases of matter” with the focus being the “effect of temperature on particle movement” and with emphasis on the fact that solids, liquids, and gases change with heating/cooling and that these physical changes are reversible (B.C. Ministry of Education, 2016).
Having said that, the curriculum does also introduce students to these concepts at the grade 2 level with the “Big Idea” that “materials can be changed through physical and chemical processes” with elaborations again given as sample questions to promote inquiry:
Why would we want to change the physical properties of an object?
What are some natural processes that involve chemical and physical changes?
Grade 2 students are expected to consider the physical ways to change materials (i.e., warming, cooling, cutting, bending, stirring, mixing), as well as the chemical ways to change materials (i.e., cooking, burning, etc.) (B.C. Ministry of Education, 2016).
In addition to this, the grade 2 science curriculum now includes the water cycle as one of its “Big Ideas” with a focus on the concept of the water cycle being “driven by the sun” as well as the concepts of evaporation, condensation, precipitation, and runoff taught (B.C. Ministry of Education, 2016).
Because students are introduced to these concepts in grade 2 and again in grade 4 (the water cycle has been removed from the grade 4 curriculum, so just the phases of matter are dealt with again), I feel that grade 4 students will be able to delve deeper into the concepts surrounding matter (solid, liquid, gas); however, as the students I teach tend to have significant difficulties in most areas of their lives, including academics, I think I would personally tend to focus on evaporation from the particulate nature of matter as I feel that might be more accessible to them at this age to grasp. Having said that, it would be interesting to design a “challenge” group activity that would integrate a macromolecular view of the process, allowing students to extend their thinking past the “concepts for all” that I would target for the class.
References:
British Columbia Ministry of Education (2016). Area of learning: Science, grade 2 and grade 4. Retrieved from https://curriculum.gov.bc.ca/curriculum/search?type%5B0%5D=big_idea&type%5B1%5D=concept_content&type%5B2%5D=curricular_competency&field_subject_range_value%5B0%5D=Science&field_grade_range_value%5B0%5D=2&field_grade_range_value%5B1%5D=4&keys=
I agree with your points about time restraints and I don’t htink this point should be overlooked. Educators, especially in the elementary panel have to teacha wide range of subject areas and are often pushed to spend most of their time on the “main” subjects of math and literacy, leaving little room for science. In addition, in the elementary school I teach at the planning teachers are responsible for treaching science, which means it is rarely integrated with other subject areas and only receives 40 minutes of time 3 days a week. Due to these time constariants the teaching model is mostly to teach the content of the science starnd, with little time for inquiry or experimentation, let alone generating, evaluating and modifying as laid out in GEM. Considering the curriculum, I believe there needs to be less focus on content in the elementary grades and more emphasis on “big ideas” and ways of problem solving in science, as well as more modelling and simulations and less pencil to paper work.
Hi Michelle,
Thank you for your response. I find it very interesting that science is taught by planning teachers! I have not actually heard of this before, but it would certainly take away from the ability to incorporate science in cross-curricular activities. I find that science can be tied in so well with other areas of the curriculum that I think I would be really disappointed to be told I could no longer teach it within my classroom. I certainly see your point in a case like yours that there is little time left for experimentation or GEM – by the time a new teacher transitions in and students are ready to start, and clean-up time is incorporated as well, I would imagine the students really only get 35 minutes of science which is just not enough time to experiment or evaluate/modify ideas on any deeper level.
I am interested in how your school is set up in terms of library, music, etc. time. Do you have specialty teachers who are used as planning teachers at all? We have our planning time while our students are in library or music, which means that all curricular content is taught by the classroom teacher.