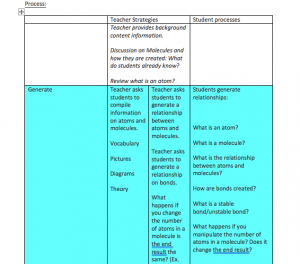
Misconception: How molecules are created and how manipulating the atoms creates new and different molecules.
*I teach elementary and would perhaps introduce this to my grade 7-8 students. It may be more applicable to high school. I explored chemistry as it is my weakest link in science so if my terminology is off I apologize to those who are chemistry stars. I just wanted to challenge myself to learn something new.
Goals:
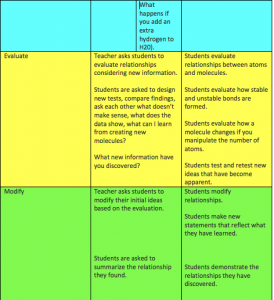
Students will be able to demonstrate basic knowledge of molecules and how they are created. Students will have an opportunity to manipulate molecules to form different compounds.
Students will recognize that molecules must be formed in a certain way with specific bonds.
Materials:
Computers on Wheels (COWS)
https://phet.colorado.edu/en/simulation/molecule-shapes-basics
One station set up with leap motion controller and molecules activity.


For a final exploration of this module or as a means to extend the learning of those who are interested I would then tie in the Molecular Workbench website. This site, although more advanced, would allow students to investigate molecules in more depth on their own.
http://mw.concord.org/modeler/
The foundation of this lesson is based on Finkelstein et al (2005) article “When learning about the real world is better done virtually: A study of substituting computer simulations for laboratory equipment “. In previous chemistry courses, students have used coloured Styrofoam and connectors to create molecules. As the students can create just about anything they have no idea if the molecules they are creating hold up to the laws of chemistry. A computer simulation would correct any misconceptions the students had. Finklestein et al (2005) reported that “results indicate that properly designed simulations used in the right contexts can be more effective educational tools than real laboratory equipment, both in developing student facility with real equipment and at fostering student conceptual understanding (p. 2).” They further state that in an inquiry-based laboratory, students using the simulations learned more content than did students using real equipment (p. 6).”
Steiff and Wilensky (2003) reported that computer-based curriculum provides an opportunity for inquiry-based chemistry lessons. (I highly suggest this article if you have not read it and teach chemistry).
“The modeling environment, connected chemistry, uses a “glass box” approach (Wilensky, 1999a) that not only enables students to visualize the molecular world but also provides them with virtually unlimited opportunities to interact with and to manipulate a simulated molecular world to gain a deeper under- standing of core chemistry concepts and phenomena (p. 285).”
Simulations allow students to make predictions about a concept and justify their predictions with observable outcomes (p. 286).
Finally, the article by Edens, K., & Potter, E. (2008) although pertaining to mathematics and word problems made me stop and really investigate the way my students use visuals to explain their thinking in every subject.
“Students who used schematic visual representations were more successful problem solvers than those pictorially representing problem elements. The more “schematic-like” the visual representation, the more successful students were at problem solution (p184).”
I realize that it would likely be beneficial for me to introduce to my students the concept of schematic visuals and pictoral visuals. Are my students drawing a picture and not really saying anything or are they using schematics to demonstrate interactions and important concepts? This idea really made me stop and think about how I have taught using visuals.
References:
Edens, K., & Potter, E. (2008). How students “unpack” the structure of a word problem: Graphic representations and problem solving. School Science and Mathematics, 108(5), 184-196
Finkelstein, N.D., Perkins, K.K., Adams, W., Kohl, P., & Podolefsky, N. (2005). When learning about the real world is better done virtually: A study of substituting computer simulations for laboratory equipment. Physics Education Research,1(1), 1-8.
Stieff, M., & Wilensky, U. (2003). Connected chemistry – Incorporating interactive simulations into the chemistry classroom. Journal of Science Education and Technology, 12(3), 285-302.
Hi Catherine,
Awesome and detailed lesson! I found it interesting, in trying out the simulation, that the bonds were somewhat elastic. I am curious (maybe a chem major and fill us in) as to how accurate this is to real life. It would make sense that molecules might de-form due to other charged compounds (are these ions???) or as they combine into new compounds.
During the generate phase, are your students working with the simulations right away or are they building some background first?
– Dan
Hi Daniel,
I would have the students explore the vocabulary of the unit first. As well as develop a basic understanding of what we are trying to discover. I think I would introduce simulations in the last portion of the Generate section. (Although students may have discovered them on their own during their discovery phase early on in the Generate section).
As for your more detailed chemistry question “ha, I have no idea” 🙂 The phet simulation I tried and the leap motion software seemed pretty straightforward to me, maybe I wasn’t trying crazy enough combinations. All I know is that I would have really appreciated having these simulations and vr when I was trying to understand chemistry.
Catherine
Hi Catherine,
Great work, as usual! This sounds like it would be a really neat lesson. I appreciate your inclusion of the Leap Motion video. I remember you talking about Leap Motion in another post, but as I have never used it, the video gave a great sample of its capabilities. I liked your lesson set-up using T-GEM to structure the lesson – as an outsider, it gave me a clear step-by-step understanding of what and how your students would be learning about molecules. I also really appreciate the attention you have drawn to visual representation of learning in your reference to the article by Edens and Potter (2008). I have not read this article, but I will put it on my list to read now. I often have my students (grade 4/5) include visuals to show their learning/thinking process. However, having read the last part of your post and reflecting back on how I have students use visuals to represent learning, I am questioning just how “schematic-like” students’ visual representations are. Your question “are my students drawing a picture and not really saying anything or are they using schematics to demonstrate interactions and important concepts?” has really made me question how I use pictorial representation of learning in my classroom, and how I can improve upon what I am currently doing. Thank you for including this and I will look further into it myself!
Mary
Bravo for trying to work in an area where it is something new: I teach elementary and would perhaps introduce this to my grade 7-8 students. It may be more applicable to high school. I explored chemistry as it is my weakest link in science so if my terminology is off I apologize to those who are chemistry stars. I just wanted to challenge myself to learn something new. i like how you started off with a misconception (although you might want to frame it as the misconception). Eg. it could be that “Students think molecules are created by X”. The literature will have lots to say on this. Alternatively, it could be stated that “Students have misconceptions about how molecules are formed (vs. created) and re-arranged to produce different molecules.” The video with Leap Motion technology helped us to see the power of this visualization tool. I also really liked how your table for your plan showed both an active teacher and an active student process at play with and without the technology. It’s great to see your reference to Concord’s Molecular workbench: http://mw.concord.org/modeler/Mike Stieff and I wrote a book together (as an fyi:Khan, S. (2013). The future of computer simulations designed for classroom instruction. In the Pedagogic Roles of Animations and Simulations in Chemistry Courses, Eds. Jerry Suits and Michael Sanger. Oxford University Press). For students interested in tinkering and with a bit of background in mathematics, it might be advantageous to explore the “glass box” nature of some of Stieff and Wilensky’s Netlogo, although Phet (a black box simulation) is also entirely appropriate here. The questions what is the relationship between atoms and molecules and the what happens if…questions are powerful ones in the generate phase (some of them like the what happens if could also be used in the evaluate phase). Down the road, it will be great to learn how the evaluation and modification activities play out in concert with the simulation. For example, is there a role for a specific aspect, test, or comparison using the simulation or leap motion in evaluating aspects of the relationship between atoms and molecules? It will be great to hear how this lesson goes and thanks for raising the point about schematics vs pictorial representations in math (and science), Samia