After reading and reflecting on the aims of LfU (Learning for Use) I believe there are a number of ways that LfU has the capability of supporting students who are experiencing conceptual challenges understanding Earth Science. The main goal of LfU experiences are to seamlessly integrate content and process activities so that students achieve robust and useful understandings that are deep and accessible (Edelson, 2001). In particular, technology supported inquiry learning provides an opportunity for these students to be supported throughout their learning. The Create-a-World Project which includes the use of the programs WorldWatcher and Progress Portfolio demonstrate a robust example of how technology can be used to support these learners. WorldWatcher provides a geographic visualization and data analysis engine whereas Progress Portfolio provides a place to record and monitor investigations and capture the ongoing work done in Worldwatcher.
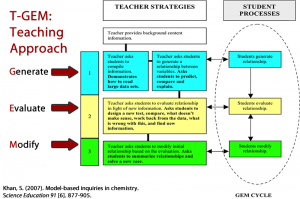
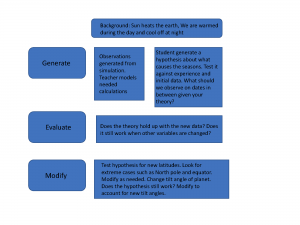
The objective of the Create-a-World Project is to have students investigate relationships between temperature and geography from a climatic perspective. Since this project is designed with the LfU model it follows certain protocols. Most importantly LFU focusses on the application of knowledge and through a knowledge application task LfU creates demand for learning and offers space for refinement as students apply knowledge they have learned (Edelson, 2001). Reflection is also built into this process and a necessary part of the learning cycle. LfU is similar to the traditional learning cycle in which students are involved in an exploration or activities that help them understand a concept. This includes hands-on observations, measurement and gathering of evidence. Through this process, students begin to explore relationships and concepts and/or discuss findings and finally additional observations are discussed, noted and shared then applied and refined.
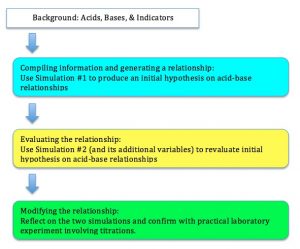
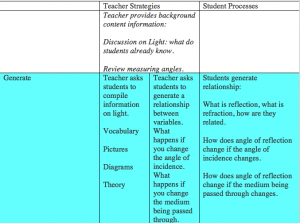
Examining a knowledge application task will illustrate the process and how technology can support the aims of LfU. In the introduction of the Create-a-World project students are inspired to begin to think about global temperature through guessing and colouring in the average temperatures in the world in July. This is to start the discussion about the concept and to promote communication. The LfU reasoning for this is to elicit curiosity and to have students confront limitations in their understandings (Edelson, 2001). It is noted in other literature that students are not likely to change their understandings in science until they notice contradictions to existing ones and that constructing relationships is a way to breach this divide (DeLaughter, Stein, Stein & Bain, 1998).
In step 2 students compare conjectures using WorldWatcher using real data. They use visualization and analysis tools to compare their own maps with actual July temperatures around the world. The LFU reasoning for this is that this allows students begin to observe patterns of temperature variation and to elicit curiosity in their causes (Edelson, 2001).
In fact, deeper more robust learning occurs when we encourage students to pursue a concept in a variety of contexts and examples until these new models are integrated. The students need to understand why they are pursuing the problem and this is best achieved when students encounter information in the context of pursuing larger problems and issues that they find intriguing (DeLaughter, et al., 1998)
In step 3 the students invent their own worlds using a paint interface and data sets. The LfU reasoning is to create a demand for student learning. Students must have an understanding of temperature to create this world.
In activity 4 students begin to explore the relationship between geography and temperature using WorldWatcher tools. The maps created are inputted into the Progress Portfolio program and they are able to annotate the relationships they see. Then they engage in group discussions in which they further refine their understandings. In this way they acquire additional knowledge construction.
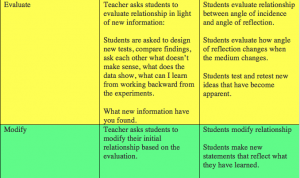
In activity 5 the students begin to explain findings through discussions and have the opportunity for hands-on laboratory explorations of concepts thus explored. At this time the teacher can offer explanations or address misconceptions.
Finally, in activity 6 the students create temperature maps for their created worlds based on all the factors they have studied. They also document the rules they are using while creating these maps and record these in their progress portfolio. Then they present to their classmates and explain their work and have an opportunity to discuss the reasoning behind their choices.
So after outlining this example, here are the ways that I believe that LfU has the capability of supporting students who are experiencing conceptual challenges understanding Earth Science. Firstly, LfU design creates demand for learning and eliciting curiosity. In the Create-a-World project the students are required to create a fictitious world, and this would be the impetus for learning about temperature and climate. The technology used in WorldWatcher allows them to paint data and manipulate data for this purpose. So technology is supporting this type of learning.
In addition, eliciting curiosity through identifying potential misconceptions and for activating existing knowledge is achieved with technology. Technology provides simulations which may be unavailable to direct observation (Edelson, 2001). Technology may also provide ways to articulate and demonstrate concepts using, for example, drawing programs. Eliciting curiosity may not happen with traditional style lecture or through textbooks which often tend to be outdated or misrepresent scientific concepts.
As students continue to discover more about scientific concepts and delve deeper with their understandings, technology can assist with data collection and analysis, modeling, and prediction which may be hampered without these technology tools due to time constraints, lack of resources or complex data management capabilities.
The computer is also used as a communication tool which provides the ability to present information in a wide variety of formats, which may not be possible in traditional presentations. This not only allows for differentiation but also allows for students choice, both aims of educational reform.
Finally, technology provides a place for reflection. It supports record-keeping during inquiry and also provides for the possibility of ongoing discussion threads for communication as well as presentation tools. In addition, investigation tools are provided through visualization and analysis capabilities, artifact construction, expressive and record keeping data collection and tools such as annotation as well as drawing capabilities.
I look forward to your reflections.
DeLaughter, J. E., Stein, S., Stein, C. A., & Bain, K. R. (1998). Preconceptions abound among students in an introductory earth science course. EOS Transactions, 79 (36), 429-436.
Edelson, (2001). Learning for use: A framework for the design of technology-supported inquiry activities. Journal of Research in Science Teaching. 38 (3), 355-385.