As I reflect on the four foundational technology-enhanced learning environments (TELE) that we’ve looked at over the past few weeks, I notice a number of similarities in the foundational focus of each of them, while also noting subtle differences in their application, teaching methods and technology integration.
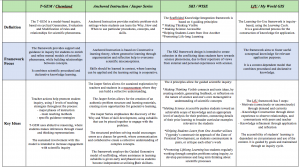

For a pdf version of the tele comparison table
Inquiry has been a contributing factor throughout my career as a teacher, and continues to be emphasized in all of these TELE’s, as they highlight the importance of inquiry in the construction of student knowledge. As we saw with the video “A Private Universe” early on, just like Heather, many students struggle with misconceptions towards scientific concepts that are not relevant to their daily lives or are inaccessible due to the unobservability of the phenomenon. Using a process of inquiry, student-driven learning supported by teacher scaffolding, and technology integration, students can overcome misconceptions and develop stronger scientific engagement and understanding.
In examining each of the TELE’s, I have gained a greater awareness of the diverse ways in which educators can support students’ conceptual understandings and begin to construct accurate representations in their minds. Technology plays a huge factor in making science accessible, whether it is through the Jasper series problem sets, simulations, or data sets using My World GIS or Google Earth. However, using technology and applying a framework to support learning around a technology are quite different. I have learned that proper integration comes with an intention. Students can’t properly learn to manipulate data in Chemland without a solid understanding of mass or temperature in the Heat Transfer Between Substances example. There must be a balance of scaffolding and open exploration, where teachers help guide students in what they are meant to observe or skills they are focusing on developing, while allowing them to explore the extremes of problems and phenomena.
Beginning this unit, we were asked to think about what we pictured as our Ideal TELE. Having explored the four foundational TELE’s, there are many attributes of each that I would apply to my own teaching practices, with the goal of students becoming lifelong learners. The anchored instruction approach presents realistic problem scenarios to create independent thinkers (Cognition & Technology Group at Vanderbilt, 1992; Shyu, H., 2000) that I would like to make accessible to my students through video-based problems such as the Jasper Series or Encore’s Vacation (Shyu, H., 2000). I also liked the integrated communication aspects of WISE that allow students to engage in critique of other students’ work to better build their own understandings. Using visual “dynamic, runnable models” to examine causal and temporal processes of WISE (Gobert, J et al., 2002) would further allow me to help students make their thinking visible, not only to myself, but to themselves. This would contribute to the process of self-reflection of whether students are constructing and refining their mental models accurately. Finally, my ideal TELE would combine the aspects the T-GEM model and LfU framework, as I see many similarities between the two already. To best support relationship generation, evaluation and modification, I would include a motivation by having students experience curiosity and demand through their knowledge gap; construct knowledge through direct observation and evaluate their knowledge with peers; finally applying and reflecting on their understanding, all the while I would be supporting their efforts through guided questioning.
Although it seems like a bit of a daunting task, combining all that we have learned through the TELE’s, the take-away for me is that educators should help guide students’ construction of knowledge through meaningful, relevant and applicable scenarios, where technology helps to enhance the learning of STEM with an intention and a reinforcement of connections that students refine through sustained scientific engagement.
References:
Cognition and Technology Group at Vanderbilt (1992a). The Jasper experiment: An exploration of issues in learning and instructional design. Educational Technology, Research and Development, 40(1), 65-80.
Edelson, D.C. (2001). Learning-for-use: A framework for the design of technology-supported inquiry activities. Journal of Research in Science Teaching,38(3), 355-385.
Gobert, J., Snyder, J., & Houghton, C. (2002, April). The influence of students’ understanding of models on model-based reasoning. Paper presented at the Annual Meeting of the American Educational Research Association (AERA), New Orleans, Louisiana.
Khan, S. (2007). Model-based inquiries in chemistry. Science Education, 91(6), 877-905.
Khan, S. (2010). New pedagogies for teaching with computer simulations. Journal of Science Education and Technology, 20(3), 215-232.
Linn, M., Clark, D., & Slotta, J. (2003). Wise design for knowledge integration. Science Education, 87(4), 517-538.
Shyu, H. Y. C. (2000). Using video‐based anchored instruction to enhance learning: Taiwan’s experience. British Journal of Educational Technology, 31(1), 57-69.
